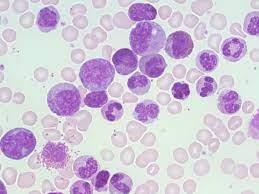
 Kembara Xtra - Medicine - Chronic Myelogenous Leukemia A myeloproliferative neoplasm called chronic myelogenous leukemia (CML) is characterized by clonal proliferation of myeloid progenitors in the bone marrow and ongoing differentiation into adult granulocytes. Philadelphia chromosome (translocation t[9;22]) is a hallmark of CML. The disease's natural course progresses through three clinical phases: a chronic phase, an accelerated phase, and a blast phase or crisis (which has the potential to progress to acute myeloid leukemia (80%) or acute lymphoblastic leukemia (20%)). Epidemiology Incidence: 1.9 cases/100,000 people annually; 65 as the median age at diagnosis; and males outnumbering females (1.7:1) Prevalence makes up between 15 and 20% of adult leukemias Pathophysiology and Etiology The BCR gene on chromosome 22 and the ABL gene on chromosome 9 are balanced translocated on the Philadelphia chromosome, or t(9;22)(q34;q11). The aberrant, constitutively active tyrosine kinase that is encoded by the fusion gene BCR-ABL impacts a number of signal transduction pathways, leading to unchecked cell proliferation and diminished apoptosis. Genetics acquirer genomic alterations Risk Elements exposure to ionizing radiation is infrequent. Diagnoses Between 85 and 90 percent of patients have a chronic condition, and up to 50 percent of cases of the disease are discovered by chance during routine screening. Chronic phase: tiredness, weight loss, sweats at night, abdominal heaviness caused by an enlarged spleen, early satiety, dyspnea, and bleeding. Bruising, pain in the left upper quadrant of the abdomen, sternal pain (caused by growing bone marrow), and gouty arthritis are uncommon; up to 30% of patients have no symptoms. Accelerated phase: increasing splenomegaly, sweating, inexplicable fever or bone pain, chloromas (extramedullary tumors), and occasionally discomfort in the left upper quadrant of the abdomen that radiates to the left shoulder (caused by splenic infarction or rupture). The acute phase is characterized by bruising, bleeding, infections, and severe constitutional symptoms. clinical assessment Hepatomegaly (10%) and splenomegaly (50–90%) Less frequent: lower sternal discomfort, lymphadenopathy, and splenic friction rub Differential diagnosis includes infectious mononucleosis, leukemoid reaction, polycythemia vera, chronic myelomonocytic leukemia, chronic neutrophilic leukemia, chronic eosinophilic leukemia, juvenile myelomonocytic leukemia, and treatment with granulocyte-stimulating agents. Acute lymphoblastic leukemia and acute myelogenous leukemia both resemble blast crises with myeloid blasts and lymphoid blasts, respectively. The clinical hematologic profile of atypical CML is similar to that of CML, however it lacks the Philadelphia chromosome and the BCR-ABL rearrangement. Dysplastic granulopoiesis is a defining characteristic. CBC - WBC count: significantly elevated (50,000 to 100,000/L), with granulocytes in all stages of maturation, occasionally (10%) blasts in chronic phase, basophilia, and eosinophilia. - Hematocrit: it could be normal, slightly elevated, or lowered. Anemia, >20% basophils, and thrombocytopenia are signs of an accelerated phase of the disease. Platelets may be normal, high, or infrequently low. Bone marrow biopsy: myeloid hyperplasia and hypercellular marrow. Blast phase is indicated by 10–19% blasts in the accelerated phase and > or = 20% blasts in the blood or bone marrow. Genetics - Cytogenetic methods, fluorescence in situ hybridization (FISH), or reverse transcription polymerase chain reaction (RT-PCR) demonstration of the Philadelphia chromosome, t(9;22). – In both the rapid and blast stages, there are additional cytogenetic abnormalities (monosomy 7, t[3,21], trisomies 8, 19, and Philadelphia chromosome duplication, as well as abnormalities of chromosome 17 such monosomy, trisomy, and isochromosome mutations). These could be a factor in tyrosine kinase inhibitor (TKI) resistance, such as imatinib. If the patient stops responding to treatment, further molecular testing (for BCR-ABL mutations) is advised. Other factors include: - Neutrophils with low or nonexistent leukocyte alkaline phosphatase - High lactate dehydrogenase (LDH) - Elevated uric acid Initial examinations (lab, imaging) Baseline RT-PCR (quantitative and qualitative analysis to determine BCR-ABL transcript type), CBC, LDH, uric acid, LFTs, bone marrow biopsy and aspiration, cytogenetics on bone marrow, FISH for BCRABL, and Splenomegaly is visible on abdominal ultrasonography or CT scans; not necessary Tests in the Future & Special Considerations RT-PCR serial monitoring of BCR-ABL is utilized to assess therapy response. Tyrosine kinase domain mutation study of the ABL kinase can forecast treatment resistance to TKIs. HLA-A*02 positivity is linked to CML, and the HLA-B*35 allele is protective (pooled odds ratio 0.64, 95% CI 0.48-0.86). Diagnostic Techniques/Other Biopsy and aspiration of the bone marrow Interpretation of Tests Increased myeloid:erythroid ratio, normal maturation, marrow basophilia, and enhanced reticulin fibrosis are all characteristics of myeloid hyperplasia. Medication TKIs (such as imatinib) offer dependable, long-lasting disease control. The response to TKIs is evaluated at particular intervals following the start of treatment and is divided into the following categories: Complete hematologic response (CHR): return to normalcy of peripheral counts, absence of illness symptoms, and absence of immature cells Major molecular response (MMR): lowered amount of BCR-ABL transcript by PCR 3-log; Complete cytogenetic response (CCR): no Philadelphiapositive metaphases on chromosomal analysis - Complete molecular response (CMR): PCR cannot detect BCR-ABL transcript. Failure to reach CHR in 3 months is a sign that therapy should be changed. – The most significant prognostic indicators or outcome predictors include hematologic, cytogenetic, and molecular responsiveness to TKIs. Initial Line Side effects of the oral TKI imatinib mesylate (Gleevec), 400 mg/day, include thrombocytopenia, anemia, increased liver enzymes, edema, GI problems, and rash. Imatinib was made the first-line treatment by the International Randomized Study of Interferon vs STI571 (IRIS), which also shown long-term efficacy after a 10-year follow-up. If the recommended amount of imatinib only produces subpar results, the dose can be increased to 600 or 800 mg/day. nilotinib (Tasigna), dasatinib (Sprycel), and bosutinib (Bosulif) are 3 examples of 2nd-generation TKIs that have been approved for use as the first-line therapy for chronic phase CML. These drugs have higher efficacy and fewer adverse effects. Next Line 2nd-generation TKIs; effective against the majority of BCR-ABL mutants; ineffective against the T315I mutation. Dasatinib: 100 mg/day in patients resistant or intolerable to imatinib; 70 mg BID or 140 mg/day for patients in the accelerated or blastic phases. Nilotinib: 400 mg PO BID in patients resistant or intolerable to imatinib in the chronic or accelerated phase. Side effects: pleural Cytopenias, QTc prolongation, pancreatitis, hyperglycemia, and a higher risk of thrombotic events are side effects. - Bosutinib 400 mg PO daily, 500 mg in patients unable to tolerate or resistant to prior treatment in the chronic or accelerated phase. GI toxicity, myelosuppression, hepatotoxicity, and fluid retention are some of the side effects. Ponatinib, a third-generation TKI, has limited FDA approval due to the possibility of thrombotic complications, yet it and omacetaxine (below) are the only treatments that are effective in treating individuals with the T315I mutation. Other authorized or in development substances: Omacetaxine is licensed for use in patients who are intolerant or resistant to two TKIs. - Patients with chronic phase CML who have received two or more TKI treatments in the past or who carry the T315I mutation are showing potential with asciminib, a STAMP inhibitor that specifically targets the ABL myristoyl pocket. QUESTIONS FOR REFERENCE A hematologist should be consulted by all CML patients. Patients with T315I mutations or insufficient TKI response should speak with a bone marrow transplant (BMT) specialist. ADVANCED THERAPIES Leukemia stem cell-targeting medications are being created for therapeutic application. PF-114, a fourth-generation oral TKI, and HQP1351, a third-generation oral TKI, are emerging drugs being researched. Operative Techniques Allogeneic BMT It is the only known cure, yet no patient progressed on the trial between years 5 and 6 of medication, and 71% of patients who achieve CCR with imatinib retain that response for more than 7 years. Most beneficial for people under 50 who are in the chronic phase Initial mortality is higher with myeloablative regimens compared to conventional care, but pre-TKI era survival rates were higher. Nonmyeloablative regimens have shown improvements in transplant-related mortality. Transplant option should be thoroughly discussed with young patients in chronic phase and considered an alternative to TKIs, especially if the patient does not tolerate TKIs or the disease is not responding. Significant improvement in transplant techniques leading to better outcomes, such as alternative sources of stem cells. Can be taken into account in patients with blast phase CML who are in remission, patients with accelerated phase CML who have a subpar or resistant response to TKIs, patients with chronic phase CML who have not achieved CHR by three months, who have not experienced cytogenetic improvement or relapse, who have the T315I mutation, or patients with extramedullary disease containing chronic phase CML cells. Admission Acute abdominal symptoms (spleen infarction or rupture), tumor lysis syndrome brought on the early therapy, and BMT complications - Hydroxyurea may be administered to lower the WBC count, although it has little effect on how well patients respond to TKIs. - Induction chemotherapy in the context of blastic phase (for acute leukemia) Discharge criteria: cessation of acute symptoms - Allopurinol to prevent tumor lysis syndrome in individuals with very high numbers; however, probably not necessary when TKIs are used Follow-Up Frequency is based on presentation stage and first-line therapeutic response. Even though splenomegaly still exists, stay away from contact sports and abdominal trauma. patient observation CBC with differential: once weekly until blood counts stabilize, then every two to four weeks during CHR; once the patient is in CCR and stable, less frequent monitoring (3-month intervals) is possible. Bone marrow cytogenetics (assessment for clonal evolution) is performed every six months while receiving CHR and every twelve to eighteen months while receiving CCR, MMR, or CMR. Quantitative RT-PCR in peripheral blood every three months LFTs when using TKIs, ECGs (QT prolongation of concern). Pancreatitis can be brought on by nilotinib, bosutinib, and ponatinib. Because numerous TKIs have been linked to high blood pressure and concomitant cardiovascular problems, blood pressure monitoring is recommended. Prognosis: With proper care and a positive reaction, the survival rate is comparable to that of the general population. Without therapy, CML will inevitably advance to the accelerated phase in 2 to 5 years and the explosion phase a few months after the accelerated phase. Patients with an accelerated or blastic phase of the disease, a very large spleen, platelets above 700,000/L, and T315I mutation resistance have a poor prognosis. Complications include bleeding caused by low or defective platelets, splenic infarct or rupture, progression to the accelerated or blastic phase, and anemia-related sequelae.
0 Comments
Leave a Reply. |
Kembara XtraFacts about medicine and its subtopic such as anatomy, physiology, biochemistry, pharmacology, medicine, pediatrics, psychiatry, obstetrics and gynecology and surgery. Categories
All
|


 RSS Feed
RSS Feed
